The mechanism by which temperature exerts its influence on offpring sex has baffled scientists for over 50 years, since the phenomenon was first discovered. In a paper recently published in Science Advances (June 14, 2017), we believe we have discovered an important piece of the puzzle, a temperature sensitive transcripional modification to an influential chromatin modifying gene, Jumonji. Here is some background to the discovery.
In the mid 1960's, a scientist in east Africa, Madeleine Charnier, incubated some eggs from the local dragon lizard, Agama agama. To her surprise, all of the offspring from eggs incubated at 30C were male whereas both male and female offspring were produced at the lower temperature of 28C.

Captialising on her "that's funny?" moment, Madeleine published her findings in the Bulletin of the Biological Society of West Africa. Her finding was not picked up immediately by the scientific community because it was so bizarre as to be met with complete disbelief. After all, it has been accepted for many decades that sex is determined by the complement of chromosomes one receives from their parents, an arrangement carefully crafted by God or nature to ensure that "every Male may have its Female, and of a proportionable Age".
We now know that a very many reptiles allow the sex of their offspring to be determined by the ambient temperatures in the nest, and that genetics has very little to say in the decision of whether they be male or female. It is true of all crocodiles, all marine turtles, many freshwater turtles and lizards, and even the Tuatara, an ancient lineage now found only in New Zealand.
What a surprise this was, and it led to a flurry of investigations showing that the offspring did not revert in some way to a "genetic sex" by adulthood, that the embryo was sensitive to thermal influence in the middle third of incubation, and that the pattern of influence of temperature varies from species to species as it is fine-tuned by natural selection to interlock with their particular life histories. Great concern is held for the future of many species with thermolabile sex because they would appear particularly vulnerable to climate change. A population of all males or all females will not have a rosy future.
Despite this attention, the mechanism by which temperature exerts its influence remains entirely mysterious. Until recently, we have had no clue. The reason for this lack of progress spanning 5 decades is because of the difficulty of disentangling the processes that make the "decision" to be one sex or the other, and the processes of differential development that follow that decision.
In a sense, animals with sex chromosomes are easier targets for science. We know in humans that boys are produced if they receive the Y chromosome from their fathers, and girls are produced if they receive the X chromosome from their fathers. This provides us with a focus. The sex determining gene must either be on the Y chromosome and act as a testis determining factor, or else by on the X chromosome where in double dose in the XX individuals directs development to be female; single dose in the XY individuals directs development to be male.
Australian researchers Andrew Sinclair and Peter Koopman pinned down the sex determining gene in mammals, Sry, by following this line of enquiry. What genes reside on the X and Y chromosomes, which of them are differentially expressed in males and females at the right time in development? Can you cause sex reversal by experimentally upregulating or downregulating the candidate sex determining genes?
The gene Sry on the Y chromosome is now widely accepted as the sex determining gene in mammals. A similar process was followed by a team led by another Australian, Craig Smith, to show that the gene DMRT1 is the sex determining gene in the chicken. In birds, it is the chromosomal complement passed on to the chicks from the mother, not the father, that determines their sex, a distinction emphasised by referring to the males as ZZ and the females as ZW. Functioning DMRT1 resides on the Z chromosome, with double dose of the gene leading to male chicks and single dose of the gene leading to female chicks. The race is on to capitalize on this new knowledge to manipulate the sex ratio of chicks produced by the poultry industry.
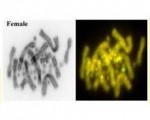
But no sex determining gene has yet been discovered in a reptile. We at the University of Canberra have been working on sex determination in the lizard, Pogona vitticeps. In a sense, we are returning to our roots by studying, like Madeline Charnier, sex determination in a dragon. And we have had our fair share of "that's funny?" moments. We found that the dragon has a ZZ/ZW system of sex determination, like in birds. What is strange though, is that this chromosomal mechanism of determining sex can be over-ridden by high temperatures, displacing ZZ individuals from their normal male developmental trajectory to become females. This is very odd, as it means that the W chromosome is not necessary for female development. It is tantamount to finding that we can produce human boys without the Y chromosome, which would be odd indeed.
Following in the tracks of Andrew Sinclair, Peter Koopman and Craig Smith, we are on the hunt for the first sex determining gene in a reptile, and have found a number of promising candidates on the Z chromosome that we are investigating now. We think we are hot on the heels of discovering the sex determining gene in the dragon!
But what about temperature-dependent sex determination? The reason that this is such a hoary chessnut is because the regulation of gene expression leading to male or female could be disrupted at so many places by temperature, it is difficult to know where to start. One could imagine a gene responsible for the construction of channels for the passage of key molecules across the nuclear cell membrane being thermosensitive, and so capable of interfering with the regulatory cascade leading to one sex over the other in a thermosensitive manner. There is no need for such a gene to be on a "sex chromosome" –- it could be anywhere. Because temperature exerts its influence over such a broad range of stages in reptiles –- the middle third of incubation –- the timing of the influence of a temperature sensitive gene is not necessarily well defined.
We have suggested that temperature dependent sex determination could be more a consensus, a parliamentary system, where the regulatory processes of sexual differentiation are displaced at many points by temperature. If that displacement persists to the timepoint when the developmental trajectory locks in to the cannalised pathways leading to one sex or the other, then sex will have been determined by temperature. Where better to look for this than in a species where there is a clear underlying genetic disposition to be one sex or the other, displaced in its developmental trajectory by temperature –- our home grown Pogona.
It is in this context that a young PhD student, Ira Deveson working with the Garvan Institute of Medical Research, made an interesting discovery. With the encouragement of postdoctoral fellow Clare Holleley, now a Research Scientist with CSIRO, Ira closely examined the transcriptomes -– expressed DNA sequences -- of normal male, normal female and sex-reversed female dragons. He found that in the lizards sex reversed by high temperatures, Jumonji chromatin modifier genes carried with them an extra segment of DNA that is normally excised when the genes are transcribed. These retained introns were present only in the dragons that had been sex reversed by temperature. That's funny, he thought, and looked wider to find the very same intron differentially retained in alligator embryos incubated at high temperatures. Also in turtles, but with the reverse pattern of differential expression. It seems that we have discovered an ancient highly conserved mechanism of differential intron retention that is in some way involved in temperature-dependent sex determination.
This latest research has just appeared in the journal Science Advances, and possibly contains the most important result to emerge from our studies of dragon sex to date. We have shown that an intron is differentially retained in mature transcripts from each of two Jumonji-family genes, JARID2 and JMJD3. JARID2 is a component of the master chromatin modifier Polycomb Repressive Complex 2. A closely related Jumonji-family member directly regulates the mammalian sex-determining gene Sry. We propose that the perturbation of JARID2/JMJD3 function by intron retention alters the epigenetic landscape to override chromosomal sex-determining cues, triggering sex-reversal at extreme temperatures. Sex-reversal may then facilitate a transition from genetic sex determination to TSD, with JARID2/JMJD3 intron retention preserved as the decisive regulatory signal.
These are exciting times, and we believe that these findings will provide focus for researchers internationally in the collective efforts to pin down the mechanisms of temperature-sensitive sex determination, mechanisms that have eluded us for so many decades. Our research has fortunately been littered with the "that's funny?" moments that sustain scientists in their work and that were the centerpiece in the writings of prolific science advocate Isaac Asimov in championing science to a generation. The opportunities in the genomic era for exploring the unknown have never been greater, and it is a fabulous time to be in science.
Further reading
Deveson, I.W., Holleley, C.E., Blackburn, J., Graves, J.A.M, Mattick, J.S., Waters, P.D. and Georges, A. 2017. Differential intron retention in Jumonji chromatin modifier genes implicated in reptile temperature-dependent sex determination. Science Advances 3(6):e1700731.
Holleley, CE, Sarre, SD, O'Meally, D. and Georges, A. 2016. Sex reversal in reptiles: reproductive oddity or powerful driver of evolutionary change? Sexual Development doi:10.1159/000450972.
Holleley, C.E., O'Meally, D., Sarre, S.D., Graves, J.A.M., Ezaz, T., Matsubara, K., Azad, B., Zhang, X. and Georges, A. 2015. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523:79-82.
Quinn, A.E., Sarre, S.D., Ezaz, T., Graves, J.A.M. and Georges, A. 2011. Evolutionary transitions between mechanisms of sex determination in vertebrates. Biology Letters 7:443-448.
Georges, A., Ezaz, T., Quinn, A.E. and Sarre, S.D. 2010. Are reptiles predisposed to temperature-dependent sex determination? Sexual Development 4:7-15.
Quinn, A.E., Georges, A., Sarre, S.D., Guarino, F., Ezaz, T., and Graves, J.A.M. 2007. Temperature sex reversal implies sex gene dosage in a reptile. Science 316:411.