Contemporary society is seeing a blurring of what we mean by gender -- male or female. More commonly than you think, this blurring extends to the biological underpinning of gender, that of biological sex, no more so than in the reptile world. Here we have genetic systems (GSD) where the male determines the sex of the offspring (XX/XY systems) as in humans, or where the female determines the sex of the offspring (ZZ/Zw systems) as in most birds, parthenogenic systems where the males are dispensed with altogether, and systems where environment not chromosomes, determines offspring sex. Reptiles provide scientists with a bonanza of opportunity to study the processes of sex determination.
Even the above categories of sex determination are not clear. Parthenogenesis (no male involvement) can be obligate, with males entirely lost from the population, or faclutative, invoked only when males are nowhere to be found, or it can be facultative and cryptic in the sense that some members of a clutch can be parthenogens and others normal sexuals. And the boundary between genetics and environment as drivers of sex can be blurred. In the central bearded dragon Pogona vitticeps, a ZZ/ZW system of sex determination can be over-ridden by high incubation temperatures to reverse the ZZ genotype to a functional female phenotype. The alpine three-lined skink Bassiana duperreyi has XX/XY chromosomes, but low incubation temperatures reverses the XX genotype to a male pheotype. Similar but distinctive in many ways, the populations of the Tasmanian skink Niveoscincus occelatus differ in having GSD in some populations, temperature-dependent sex determination (TSD), in others.
In this study, which recently appeared in PLoS Genetics, we examine gene regulation in the embryonic gonads of Pogona vitticeps where female sex is determined by chromosomes and compare that to regulation where female sex is determined by environmental temperature. Temperature captures the developmental program of sex in a way that is clearly evident in gene expression profiles, giving insight into how temperature is sensed by the cell and how that signal then propagates down the cascade to produce an ovary in animal that would otherwise be destined to be male. We compare this regulatory cascade with that generated in normal females where it is driven by the sex chromosomes.
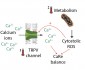
Without going into the detail (you can read the full paper for yourself), our results are consistent with a recent hypothesis we proposed, that conserved cellular mechanisms calcium flux and redox production sense and capture the temperature signal. Once the calxium-redox balance is altered, this comes to alter gene expression and regulatory pathways likely to be involved in driving sexual development. So ancient and ubiquitous cellular processes for responding to environmental stressors are enlisted to deliver the developmental reprogramming necessary to shift the sex determining cascade from a male to a female trajectory.
The challenge now is to follow the influence initiated by temperature on the cellular calcium-redox balance through ubiquitous singalling pathways to epigenetic modifications that govern the release of key sex determining genes such as AMH and DMRT1. We have already demonstrated a role for thermosensitive intron retention in key chromatin modifier genes, and other labs have done some outstanding work to show how these chromatin modifier genes regulate, through demethylation of promoter sites, the expression of sex determining genes in a TSD turtle.
Listen to Sarah Whiteley talk about this research here.
Combining this latest work (op cit), this is where we are at:
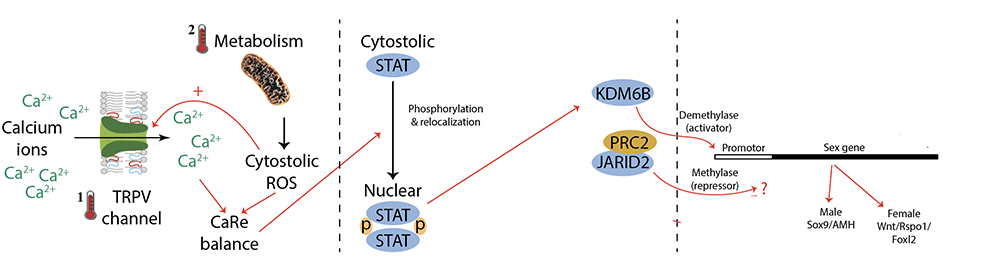
We feel we are on the cusp of something big here ..... watch this space.
Whiteley, S.L., Holleley, C.E., Blackburn, J., Deveson, I.W., Wagner, S., Graves, J.A.M., Georges, A. 2021. Two transcriptionally distinct pathways drive female development in a reptile with genetic sex determination and temperature induced sex reversal. PLoS Genetics April 15, 2021.